The energy of a photon of wavelength 500 nm is
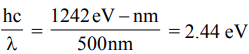
The energy of a photon of wavelength 700 nm is

The energy difference between the states involved in the transition should, therefore, be between 1.77 eV and 2.44 eV.
Figure shows some of the energies of hydrogen states. It is clear that only those transitions which and at n = 2 may emit photons of energy between 1.77 eV and 2.44 eV. Out of these only n = 3 to n = 2 falls in the proper range. The energy of the photon emitted in the transition n = 3 to n= 2 is Vector ΔE = (3.4 – 1.5)eV= 1.9 eV. The wavelength is
