Ionic hydrides are formed by combining of alkali metals or alkaline earth metals with hydrogen. In relation to the conditions in the task, there will be an alkali metal (MI) as well as an alkaline earth metal (MII) in the mixture.
Equations
(1) MI + 1/2 H2 → MIH
(2) MII + H2 → MIIH2
(3) MIH + H2O → MIOH + H2
(4) MIIH2 + 2 H2O → MII(OH)2 + 2H2
reacted: 0.09 g H2O, i. e. 0.005 mol unreacted: 0.18 g H2O, i. e. 0.01 mol Since all hydroxides of alkali metals are readily soluble in water, the undissolved precipitate is MII(OH)2, however, it is slightly soluble in water, too. Thus, the mass of hydroxides dissolved in the solution:
(5) m'(MIOH + MII(OH)2) = Z
Therefore:
30 = Z/Z+0.18 x 100
Z = 0.077 g
(6) m'(MIOH + MII(OH)2) = 0.077 g
It represents 40.95 % of the total mass of the hydroxides, i. e. the total mass of hydroxides is as follows:
(7) m'(MIOH + MII(OH)2) = 0.077g x 100/40.95 = 0 188 g
The mass of solid MII(OH)2 :

Precipitation with (NH4CO3):
(11) Ca(OH)2 + (NH4)2CO3 → CaCO3 + 2 NH3 + 2 H2O According to (5) and (6) the mass of the solution was: 0.18 g + 0.077 g = 0.257 g After precipitation with (NH4)2CO3 :
16 .81 m(MIOH)/m(solution) x 100
Let us mark as n' the amount of substance of Ca(OH)2 being present in the solution. M(Ca(OH)2) = 74 g mol-1
Taking into account the condition in the task as well as equation (11), we get:
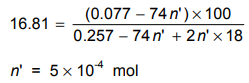
The total amount of substance of Ca(OH)2 (both in the precipitate and in the solution):
(12) (Ca(OH) ) = 0.111 g/74 g mol-1 + 5 x10-4 mol 0.002 mol (i. e. 0.148 g)
According to equations (3) and (4):
n(H2O) = 0.004 mol (for MIIH2)
n(H2O) = 0.001 mol (for MIH)
n(MIOH) = 0.001 mol
According to equations (7) and (11):

Composition of the mixture: 0.002 mol Ca + 0.001 mol Na
or 0.080 g Ca + 0.023 g Na