(a) Roasting: It is a process in which sulphide ore is heated in the presence of oxygen to convert into oxide.
2ZnS + 3O2 →2ZnO +2SO2
Calcination: It is a process in which carbonate ore is heated in the absence of air to form oxide.
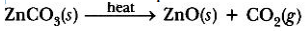
By reduction process, Zn can be extracted from its ore.

(b) Aluminium, Magnesium