The balanced chemical reactions (ionic reactions)for reduction of K2Cr2O7 by Sn2+ are:
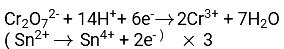
Balanced Net reaction : 3Sn2++ Cr2O72- + 14H+ → 3Sn4+ + 2Cr3+ + 7H2O
Thus according to the balanced reaction : 1 mole of Cr2O72- will be reduced by 3 moles of Sn2+
Thus 1 mole of Sn2+ will reduce = 1/3 moles of Cr2O72-
= 0.33 moles of Cr2O72-