Calculate the energy released if U238 –nucleus emits an –particle.
Calculate the energy released in MeV in the following nuclear reaction
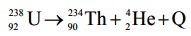
Given Atomic mass of 238U = 238.05079 u
Atomic mass of 234Th = 234.04363 u
Atomic mass of alpha particle = 4.00260 u
Is the decay spontaneous, give reason.