Correct answer is (B) I, II
K4[Mn(CN)6].3H2O ⇒Mn2+ with CN = 6 & SFL
∴ Pairing will take place 
⇒ Low spin complex with one unpaired electron.
K4[Mn(SCN)6] ⇒ Mn2+ with CN = 6 & WFL
∴ Pairing will not take place 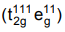
⇒ High spin complex with five unpaired electrons.
So, only II statements is correct, which matches with none of given options.
So, question should be BONUS
However, from the available options, (B) seem best.