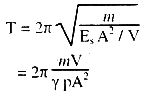(i) When the ball of mass 'm' is depressed by a small distance y, let the volume of air decreases by W Δv while pressure increase by Δp.
For isothermal change, according to Boyle's law,
pV = (p + Δp)(V - Δv)
or, pV = pV + ΔpV - pΔv - ΔpΔv
For Negative pv, we have
pΔv = ΔpV
or, p = {ΔpV}/{Δv} = {Δp}/{Δv/V}
= Stress/Volume strain
= ET [isothermal bulk modulus]
We have Stress = Δp = ET Δv/V
or, Force/Area = ET Δv/V
or, F/A = FTΔv/V ⇒ F = FTΔvA/V
or, F = FTΔvA/V
But, change in volume
v = Ay
Hence, F = ETA2/V y
Comparing it with F = Ky, we get
F = ETA2/V
Hence, the time period,
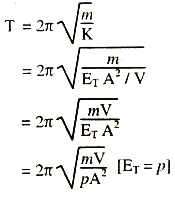
(ii) For adiabatic change, according to Poisson's law,
pV = (p + Δp) (V - Δv)'
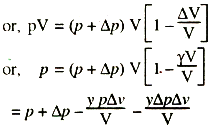
Neglecting γΔpv/V, we get
γΔpΔv/V = Δp
γp = {y}/{v/V} = {Stress}/{Volume strain} = Es (Adiabatic Bulk modulus)
∴ Es = γΔp
Again the time period is similarly given by