A graph of vapour pressure and temperature for three different liquids X, Y and Z is shown below :
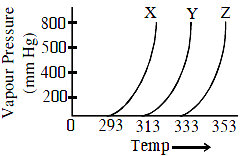
The following inferences are made :
(A) X has higher intermolecular interactions compared to Y.
(B) X has lower intermolecular interactions compared to Y.
(C) Z has lower intermolecular interactions compared to Y.
The correct inference(s) is/are:
(1) A
(2) (C)
(3) (B)
(4) (A) and (C)