Solution x
Step-1: Assign oxidation number:
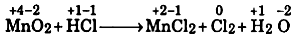
Step-2: Write the oxidation number changes.
Change in oxidation number of Mn + 4 to +2 = 2
Change in oxidation number of Cl - 1 to 0 = +1
Step-3: Cross multiply the numbers. Co-efficient 2 is multiplied to HCl and 1 is multiplied to MnO2.
MnO2 + 2HCl → MnCl2 + H2O + Cl2
Step-4: Check the oxidation number, and 2 molecules of HCl on left hand side to balance the chlorine atoms of MnCl2. In order to balance oxygen and hydrogen atom 2 molecule of H2O has to be added on the right hand side.
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2