(a) r = 0.53 A
= 0.53 x 1010m
Charge of electron =-e
Charge of proton = e
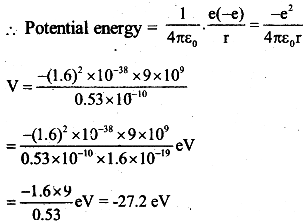
(b)Total energy = -27.2eV + 13.6 eV = -13.6eV The work required 13.6eV
(c) At 1.06 A distance, PE = -13.6eV
It is brought to zero by adding + 13.6eV
∴ PE at 0.53 is – 13.6 eV and energy needed is 13.6eV.