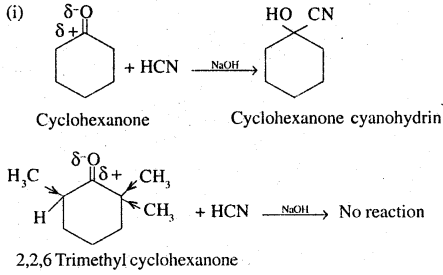
This is due to steric hindrance and +1 effect of three methyl groups in 2,2,6 - trimethyl cyclohexane. Addition of HCN to >C = O group is an example of nucleophile addition in which the nucleophile ie :CN attacks the carbon of carbonyl group. The presence of 3 methyl groups at ∝ position w.r.t. >C = O group leads to steric hindrance and reduction of positive charge on the carbonyl carbon,
2. Semicarbide is resonance hybrid of following structures.
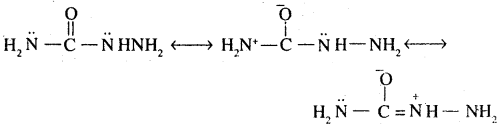
Although semicarbide has 2 -NH2 groups but one of thefn (i.e. which is directly attached to >C = O group) is involved in resonance as shown. As a result, electron density on this NH2 group decreases and hence it does not act as a nucleophile. In contrast, the lone pair of e’ on the other NH2 group (i.e. attached to NH) is not involved in resonance and hence is available for nucleophilic attack on carbon of the carbonyl group of aldehydes and ketones.
3. The formation of the esters from a carboxylic acid and an alcohol in presence of an acid catalyst is a reversible reaction

To shift the equilibrium in the forward direction, the water or the ester formed (which ever is more volatile) should be removed as fast as it is formed.