(a) Unimolecular Nucleophilic Substitution or SN1
(i) These reactions proceed in two steps:
(ii) In step I:
Heterolytic cleavage of C-X forms a carbocation (carbonium ion) (R⊕) in the form of intermediate and a halide ion X–. Step I is the slowest step, therefore, it is rate determining step.
R-X \(\overset{step}{\underset{slow}{\longrightarrow}}\) R⊕ + X–
(iii) In the first step, only alkyl halide is used therefore the rate of reaction depends only upon the concentration of alkyl halide.
Rate α [ R-X ]
Therefore, this reaction is known as unimolecular nucleophilic substitution reaction (SN1).
(iv) In the second step, carbon cation is attacked by nucleophile to form a product. Rate of the second step is much more than the first step.

(v) Like the stability of carbocation formed in step I increases, the reaction will easily proceed. Order of stability of carbocation is as follows:
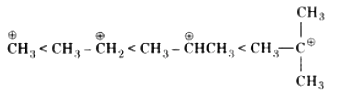
Order of reactivity of SN1 mechanism if halogen atom is the same.

The intermediate carbocation is planar, therefore attack of the nucleophile may be accomplished from either side resulting in a mixture product. Therefore if the reactant is optical isomer then the product is a racemic mixture. Mechanism of SN1 reaction between tertiary butyl chloride and aqueous KOH can be explained in the following ways:

The overall reaction can be represented as follows:
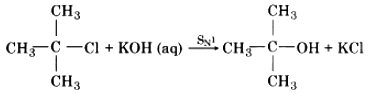
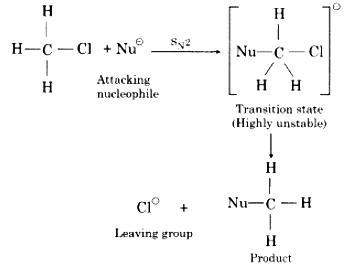
(b) Bimolecular Nucleophilic Substitution or SN2
(i) This type of reaction occurs in one step.
(ii) In this type of reaction the only formation of transition state occurs and no intermediate is formed.
(iii) For the formation of the transition state, the attacking nucleophile attacks from the opposite directions at from 180° angle of leaving the group (X®). This is known as backside attack or rear attack.
(iv) In the transition, state attacking nucleophile (Nu–) and leaving the group (X–) both are partially attacked with the central carbon atom.
(v) The rate of reaction depends upon the concentration of both the alkyl halide and the nucleophile. Therefore, these reactions are known as bimolecular nucleophilic substitution reaction (SN2).
Rate α [ R-X ] [ Nu– ]
(vi) In these reactions, the configuration of products is opposite to that reactants, therefore in these reactions inversion of configuration occurs. This is’ known as “Walden inversion”.
(vii) In these reactions, the presence of bulky alkyl group on the carbon of C-X bond produces steric hindrance for the attack of the nucleophile. Therefore with the increase in the number of alkyl groups on carbon, the reactivity of R-X decreases. If the halogen atom is same then the order of reactivity of alkyl halides towards SN2 mechanism is as:

Therefore, primary alkyl halides react more rapidly by mechanism whereas tertiary alkyl halide reacts by the SN2 mechanism. Secondary alkyl halides will react with SN1and SN2 mechanism. This depends upon the nature of nucleophile and solvent.