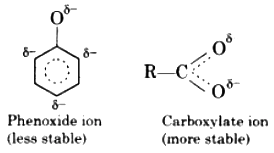
Carboxylic acids are stronger acids than phenols. It can be understood by comparing the hybrid structures of carboxylate ion and phenoxide ion. In carboxylate ion, the negative charge is equally distributed over two negatively charged oxygen atoms while in phenoxide ion it is present only on one oxygen atom. Thus carboxylate ion is more stabilised as compared to phenoxide ion. Hence carboxylic acids ionize to the greater extent than phenols furnishing higher concentration of H+ ions. Therefore carboxylic acids behave as stronger acids than phenols.