Normally, matter exists in three states: solid, liquid and gas. A change from one of these states to another can be done. These changes take place when there is exchange of heat between the substance and its surroundings.
Take some ice-cubes in a beaker. Note the temperature of ice. Slowly heat it. Observe the temperature after every minute and stir the mixture of water and ice continuously. We will see that there is no change in temperature as long as there is ice in the beaker.

In this process, even though the heat is continuously supplied, but the temperature does not change. The heat supplied is used in changing the state from solid to liquid (ice → water).
The change of state from solid to liquid is called melting and that from liquid to solid is called fusion.
The temperature remains 0°C until all the ice melts. At this point, further heat transfer will produce a temperature increase of liquid phase till it reaches 100°C, the boiling point of water. It remains at 100°C till all the water changes into gas (steam).
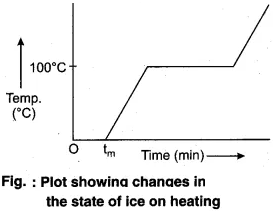
Melting Point: The temperature at which the solid and liquid state of substance (in thermal equilibrium) coexist with each other is called its melting point. The melting point of a substance depends on the nature of the substance. It also depends upon the pressure.
Boiling Point: The temperature at which the liquid and the vapour states of the substance coexist.
Sublimation: It is the change from solid to vapour state without passing through the liquid state. The substances showing sublimation are called sublime. For e.g., Dry ice (solid CO2) and iodine. In this process, both solid-state and gaseous state co-exist.