Kjeldahl Method - This method is used for estimation of nitrogen. It is used only for those organic compounds that are converted quantitatively to ammonium sulphate on heating strongly with concentrated H2SO4 .This method can not be used for the organic compounds having nitrogen in the ring e.g., pyridine and also —NO2 and —N = N— groups.
This method involves following steps :
(i) Digestion- A known mass (about 0.5 g) of the given organic compound is digested with concentrated H2SO4 in the presence of small amount of potassium sulphate and copper sulphate in a Kjeldahl’s flask. Potassium sulphate raises the boiling point of sulphric acid and copper sulphate catalyzes the digestion. In 3 to 4 hrs, the organic compound is completely decomposed to form ammonium sulphate.

Distillation- The digested reaction mixture, on cooling, is transferred to a round bottomed distillation flask, and distilled with a concentrated alkali solution (NaOH). Ammonia produced is absorbed in a known volume of HCl solution of a known strength.

The unneutralised HCl is then back-titrated against a standard alkali. From the acid consumed, the amount of ammonia produced and hence the mass of nitrogen is calculated.

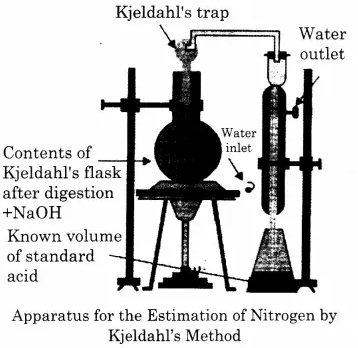
Calculations : Let,
Weight of the organic compound = W g
Volume of the standard acid required for complete neutralization of the evolved ammonia = V ml
Normality of the standard solution of acid = N
From the law of equivalence (normality equation), 1000 ml of 1 N acid = 1000 ml of 1 N NH3
= 17 g NH3 = 14 g Nitrogen
Then,
V ml of N acid = V ml of NH3
NV milli equivalent of acid = NV milli equivalent of ammonia.
Therefore, mass of nitrogen in the evolved ammonia
\(= \frac{14 \times N \times V}{1000} g\)
Then, percentage of nitrogen in the sample

Duma’s method- By this method, percentage of nitrogen in all the compounds can be estimated. A known mass of the organic compound is heated with cupric oxide in an atmosphere of carbon dioxide. The carbon and hydrogen in the compound are oxidised to carbon dioxide and water respectively, while nitrogen is set free. Any oxide of nitrogen produced during this process, is reduced back to free nitrogen by heated copper gauze. The gaseous mixture consisting of CO2, H2O and N2 is collected over an aqueous solution of potassium hydroxide. All the gases except nitrogen are absorbed by the solution. The volume of gas (nitrogen) collected is measured. From the volume of nitrogen obtained the percentage of nitrogen in the compound is calculated.

Calculations : Let,
The mass of the organic compound taken be = W g
Volume of nitrogen collected = V1 ml
Atmospheric pressure = P mm Hg
Temperature at which gas is collected = T1 K
Therefore,
Pressure of the N2 gas.P1 = (P – p) mm Hg
