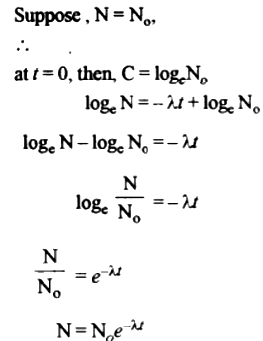Law of radioactive disintegration:
The rate of decay of radioactive atoms at any instant is proportional to the number of atoms present at that instant. Let ‘N’ be no. of atoms present in radioactive substance at any instant ‘t’. Let dN be the no. that disintegrates in a short interval ‘dt’. Then, the rate of disintegration -dN/dt is proportional to N, i.e., \(-\frac{dN}{dt}\) = λN
λ is a constant of proportionality called ' decay constant'.
\(-\frac{dN}{dt}\) = λdt
Integrating, loge N = λt + C [C is integration constant]