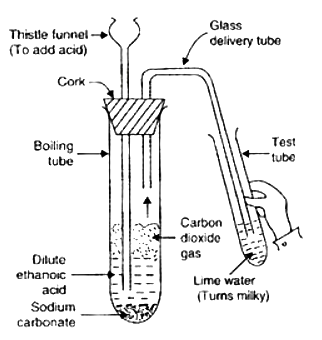
Aim: To show acid reacts with metal carbonate to liberate carbon dioxide,
Material Required: Na2CO3, Woulfe-bottle, thistle funnel, dil. HCl, gas jar, matchbox, delivery tube bent at two right angles, lime water.
Procedure:
- Take two test tubes, label them as A and B. Take about 0.5 g of sodium carbonate (Na2CO3) in test tube A and about 0.5 g of sodium hydrogen carbonate (NaHCO3) in test tube B.
- Add about 2 mL of dilute ethanoic to both the test tubes.
- Pass the gas produced in each case through lime water (calcium hydroxide solution) as shown in below figure and record your observations.
Reactions involved:
2NaHCO3 + H2SO4 → NaSO4 + 2H2O + 2CO2
Na2CO3+ H2SO4→ Na2SO4+ H2O + CO2
when ethanoic acid reacts with sodium carbonate, then carbon dioxide gas is evolved.