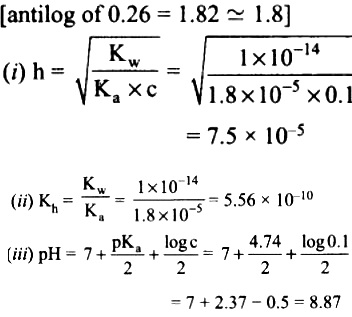CH3COONa is a salt of weak acid (CH3COOH) and a strong base (NaOH). Hence, the solutions is alkaline due to hydrolysis.
CH3COO (aq) + H2O (aq) ⇌ CH3COOH (aq) + OH- (aq)
Give that pKa = 4.74
pKa = – log Ka
i.e., Ka = antilog of ( – PKa)
= antilog of ( – 4.74)
= antilog of( – 5 + 0.26)
= 1.8 x 10-5