1. It is impossible to measure the emf of a single electrode, but we can H2 out H2 measure the potential difference between two electrodes (Ecell) using a voltmeter.
2. To calculate the emf of a single electrode, we need a reference electrode whose emf is known. For that purpose, Standard Hydrogen Electrode (SHE) is used as the reference electrode.
3. SHE has been assigned an orbitary emf of exactly zero volt.
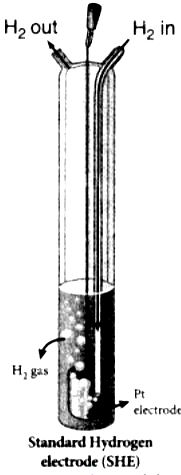
4. It consists of platinum electrode in contact with 1M HC1 solution and 1 atm hydrogen gas.
5. The hydrogen gas bubbled through the solution at 25°C. SHE can act as cathode as well as an anode.
6. The Half cell reactions are given below: If SHE is used as a cathode, the reduction reaction is
2H2 + 2e- + H2(g,1 atm) E° = 0 volt
If SHE is used as an anode, the oxidation reaction is
H2(g,1 atm) 2H+(aq 1M) + 2e- E° = 0 volt.