The intermediate compound formation theory: A catalyst acts by providing a new path with low energy of activation. in homogeneous catalysed reactions a catalyst may combine with one or more reactant to form an intermediate which reacts with other reactant or decompose to give products and the catalyst is regenerated.
Consider the reactions:
A + B → AB …… (1)
A + C → AC (intermediate) ….. (2)
C is the catalyst
AC + B → AB + C …… (3)
Activation energies for the reactions (2) and (3) are lowered compared to that of (1). Hence the formation and decomposition of the intermediate accelerate the rate of the reaction.
Example:
The mechanIsm of Fridel crafts reaction is given below

The action of catalyst is explained as follows
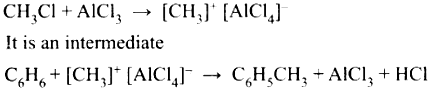
This theory describes,
1. The specificity of a catalyst.
2. The increase in the rate of the reaction with increase in the concentration of a catalyst.
Limitations
1. The intermediate compound theory fails to explain the action of catalytic poison and activators (promoters.
2. This theory is unable to explain the mechanism of heterogeneous catalysed reactions.