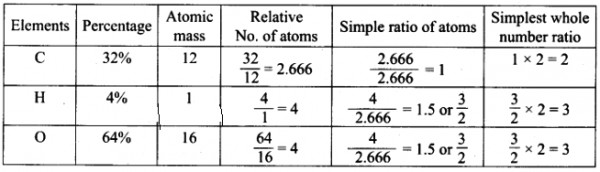
Empirical formula = C2H3O3
Empirical formula mass = 24 + 3 + 48 = 75
Molecular mass = 2 x (Vapour density)
= 2 x 75
= 150
n = (Molecular mass) / (Empirical formula mass)
= 150/75
= 2
Molecular formula = (Empirical formula)n
Molecular formula = C2H3O3 x 2
Molecular formula = C4H6O6