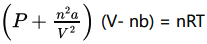
Where n is the number of moles present and ‘a’’ b’ are known as van der Waals constants.
Significance of Van der Waals constants:
Van der Waals constant ‘a’: ‘a’ is related to the magnitude of the attractive forces among the molecules of a particular gas. Greater the value of’a’ , more will be the attractive forces.
Unit of’a’ = L2 mol-2
Van der Waals constant ‘b’: ‘b’ determines the volume occupied by the gas molecules which depends upon size of molecule.
Unit of ‘b’ = L mol-1