1. Potassium combine with CO2 to form potassium carbonate.
4K + 3CO → 2K2CO3 + C
2. When a limited amount of CO2 is passed through lime water, it turns milky due to the formation of insoluble calcium carbonate.
Ca(OH)2 + CO2 → CaCO3 + H2O
(Calcium carbonate)
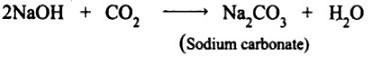
3. Sodium hydroxide (base) is neutralized by carbon dioxide (acidic) to form sodium carbonate (salt) and water.
Base + Acid ⟶ Salt + Water