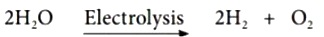The process of breaking down of water molecules by the passage of electric current is known as electrolysis of water.
Electrolysis of Water:
1. A glass beaker is fixed with two carbon electrodes and it is filled with water up to one third of its volume.
2. The positive carbon electrode acts as anode and the negative carbon electrode acts as cathode.
3. Two test tubes are placed on the electrodes.
4. The electrodes are connected to a battery’ and current is passed until the test tubes are filled with a particular gas.
5. If the gas collected is tested using a burning splint we can notice that the gas in cathode side bums with a popping sound when the burning splint is brought near the mouth of the test tube.
6. This property is usually shown by hydrogen gas and so it is confirmed that the gas inside the test tube is hydrogen.
7. The burning splint placed near the anode side bums more brightly confirming that it is oxygen gas. This experiment shows that water is made up of hydrogen and oxygen.
8. The ratio of hydrogen and oxygen is 2:1. Hence, for every two volumes of hydrogen collected at the cathode, there is one volume of oxygen collected at the anode.