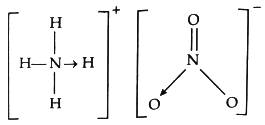
NH4+ ion contains three covalent bonds and one dative bond (formed by the donation of lone pair of electrons on N in NH3 to H+ ion). NO3− ion contains covalent and dative bonds. The bond between NH4+ and NO3− is ionic. N of NH4+ is sp3 hybridised and the shape of NH4+ ion is tetrahedral. N of NO3− ion is sp3 hybridised and the shape of NO3− ion is planar.