Given :
2A + 2B → 2C + D

If order of the reaction is x in A and y in B then, by rate law,
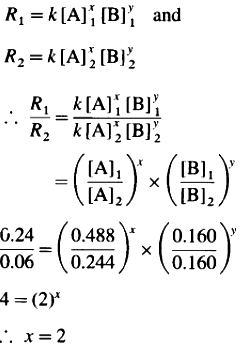
Hence the reaction is 2nd order in A.
If,

Hence the reaction is first order in B.
The order of overall reaction = n = nA + nB
= 2 + 1 = 3
By rate law,
Rate = R = k[A]2[B]
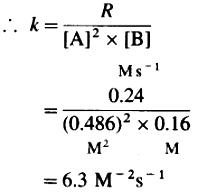
∴ (i) Order of reaction = 3
(ii) Rate constant = k = 63M-2s-1
(iii) Rate law : Rate = k [A]2[B]