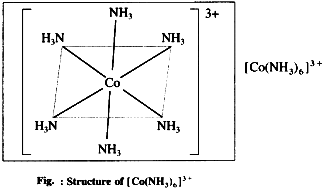
(1) Hexaamminecobalt (III) ion,
[CO(NH3)6]3+ is a cationic complex, the oxidation state of cobalt is + 3 and the coordination number is 6.
(2) Electronic configuration : 27CO[Ar]183d74s2
Electronic configuration : CO3+[Ar]183d64s°4p°

(3) Since NH3 is a strong ligand, due to spin pairing effect,
All the four unpaired electrons in 3d orbital are paired giving two vacant 3d orbitals.
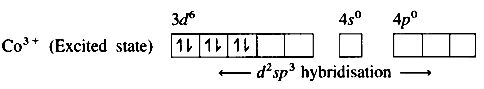
(4) Since the coordination number is Co3+ion gets six vacant orbitais by hybridisation of two 3d vacant orbitais,
One 4s and three 4p orbitais forming six d2 sp3 hybrid orbitais giving octahedral geometly. It is an inner complex.

(5) 6 lone pairs of electrons from 6NH3 ligands are accommodated in the six vacant d2sp3 hybrid orbitals.
Thus,
Six hybrid orbitals of Co3+ overlap with filled orbitals of NH3 forming 6 coordinate bonds giving octahedral geometry to the complex.

Since the complex has all electrons paired, it is diamagnetic.