Correct Option (d) benzylamine
Explanation:
Basic nature of the compound is related to their tendency to donate their lone pair of electrons more readily. -/ effect [e- withdrawing] exerting group decreases the basic strength while + / effect [e- donating] exerting group increases the basic strength of the compound.
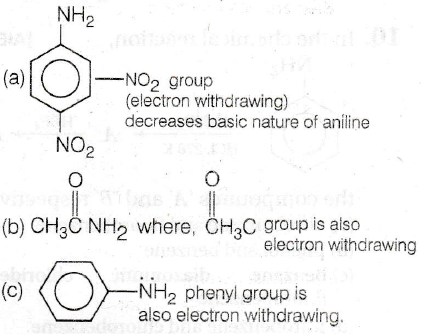
(d) Benzylamin (C6H5CH2NH2) contains alkyl group linked to amine - NH2 group. This atkyl group is + | effect [e- donating] exerting group which increases the basicity of benzylamine.
Thus, most basic compound is benzylamine.