Correct Option (B) 756.90 mm
Explanation:
Vapour pressure of pure water (solvent) at 100°C, p0 = 760 mm.
Vapour pressure of solution, p = ?
Wt. of solvent , W = 33g
Wt. of solute , w = 3g
Mol. wt. of water (H2O), M = 18
Mol. wt. of sugar (C12H22O11),
m = 12 x 12 + 22 x 1 + 11 x 16 = 342
According to Raoult’s law ,
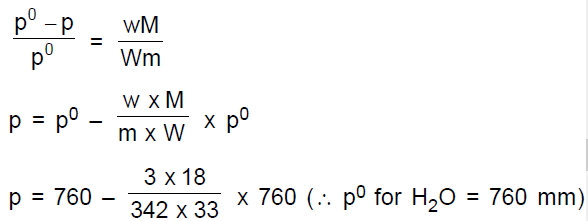
= 760 – 3.19 = 756.90mm