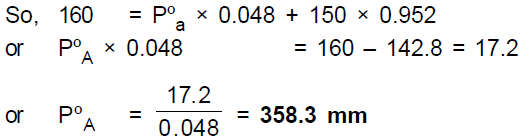Correct Option (C) 358.3 mm
Explanation:
We know that for a mixture of two miscible liquids, Ptotal = PoA .xA + PoB .xB where xA and xB are mole fractions of liquids A and B, and PoA and PoB are their vapour pressure in pure state.
Moles of liquid ‘A’ = 28/140 = 0.2
Moles of water (liquid B) = 72/18 = 4
Total number of moles in the solution = 4 + 0.2 = 4.2
Moles fraction of A = 0.2/4.2 - 0.048
Mole fraction of water (B) = 1 – 0.048 = 0.952
Ptotal = Vapour pressure of the solution = 160 mm(given)