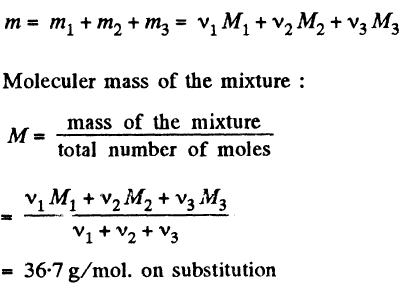(a) The mixture contains v1 , v2 and v3 moles of O2, N2 CO2 respectively. Then the total number of moles of the mixture
v = v1+v2+v3
we know, ideal gas equation for the mixture
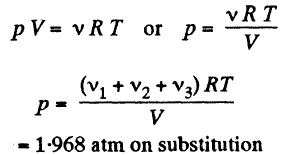
(b) Mass of oxygen (O2) present in the mixture : m1 = v1 M1
Mass of nitrogen (N2) present in the mixture : m2 = v2 M2
Mass of carbon dioxide (CO2) present in the mixture : m3 = v3 M3
So, mass of the mixture