(a) CO2(O - C - O)
The molecule has 9 degrees of freedom 3 for each atom. This means that it can have up to nine frequencies. 3 degrees of freedom correspond to rigid translation, the frequency associated with this is zero as the potential energy of the system can not change under rigid translation. The P.E. will not change under rotations about axes passing through the Catom and perpendicular to the O - C - O line. Thus there can be at most four non zero frequencies. We must look for modes different from the above

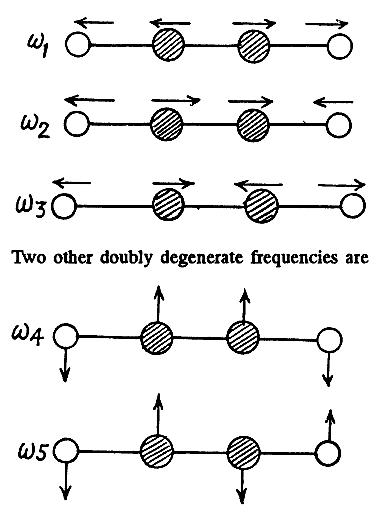
together with their counterparts in the plane ⟂r to the paper.