From the following bond energies:
H - H bond energy : 431.37 kJ mol-1
C = C bond energy : 606.10 kJ mol-1
C - C bond energy : 336.49 kJ mol-1
C - H bond energy : 410.50 kJ mol -1
Enthalpy for the reaction.
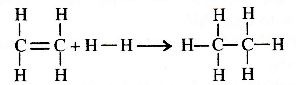
will be
(a) - 243.6 kJ mol-1
(b) -120.0 kJ mol-1
(c) 553.0 kJ mol-1
(d) 1523.6 kJ mol-1