Given: [HI(g)] = 0.85 mol dm-3
Kc = 54 at 700 K
Equilibrium concentrations of H2 and I2
Formula: Kc = \(\frac{[C]^c [D]^d}{[A]^a [B]^b}\)
Balanced chemical reaction: 2HI(g)
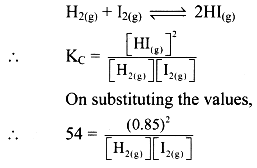
Equilibrium concentration of I2(g) = Equilibrium concentration of H2(g)

Equilibrium concentrations of H2 and I2 are equal to 0.12 mol dm-3