The correct option (a) Geometrical and ionisation
Explanation:
Octahedral Co(NH3)4Br2Cl shows ionisation and geometrical isomerism.
In ionisation isomerism ligands shown in coordination sphere and the anions present outside the coordination sphere are exchanged with each other as follows
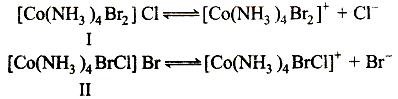
Coordination number of central atom (cobalt) is six and shape is octahedral, so it shows following geometrical isomers
