
(i) Final temperature, T2 :
In this process, W = 0, Q = 0
∴ The steady flow equation may be written as
H1 = H2
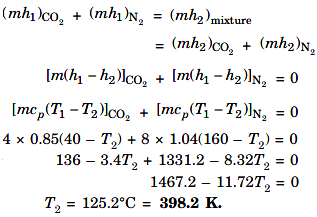
(ii) Change in entropy :
nCO2 = \(\cfrac4{44}\) = 0.0909
nN2 = \(\cfrac8{28}\) = 0.2857
n = nCO2 + nN2 = 0.0909 + 0.2857 = 0.3766
\(\cfrac{p(CO_2)_2}{p_2}\) = xCO2
[p2 = pressure of the mixture]

∴ Change in entropy, ∆S

= 4(0.2046 + 0.3984) + 8(– 0.0871 + 0.1885) = 3.2232 kJ/K
Change in entropy = 3.2232 kJ/K.