Let us draw the required structures for carboxylic acids.
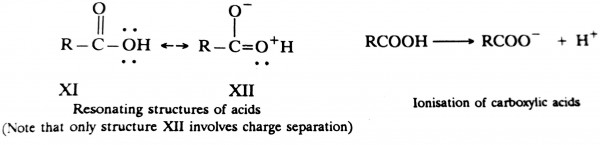

(Note that both structures are similar and hence equally stable) Note that the resonating structures XI and XII of the carboxylic acid are non-equivalent and hence much less stable than the equivalent resonating structures XIII and XIV for the carboxylate ion.
Hence carboxylic acids have a tendency to undergo ionisation to form more stable carboxylate ions and protons.
Now let us compare the acidic strengths of carboxylic acids and phenols. The resonating structures VI to X of the phenoxide ion are not equivalent, while the resonating structures XIII and XIV of the carboxylate anions are equivalent.
Hence the resonance hybrid of the carboxylate anion is relatively more stable than the resonance hybrid of the phenoxide ion.
Thus a carboxylic acid is more acidic than a phenol.