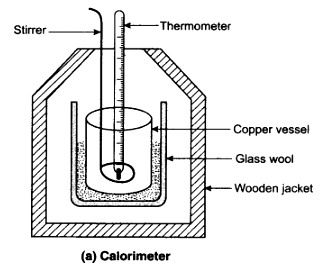
A device in which heat measurement can be done is called a calorimeter. It consists of a cylindrical vessels of copper or aluminium. The vessel is kept inside a wooden jacket which contains heat insulating material, like glass wool etc. This outer jacket acts as heat shield and reduces the heat loss from the inner vessel. There is an opening in the outer jacket through which a mercury thermometer can be inserted into the calorimeter. The lid is provided with holes for inserting a thermometer and a stirrer into the calorimeter.
When bodies at different temperatures are mixed together in the calorimeter, heat is excharged between the bodies as well as with calorimeter. If there is no heat lost to the surroundings, then according to the calorimetry principle.
Heat gained by cold bodies Heat lost by hot bodies. From this equation we can determine the specific heat and latent heat of different bodies.