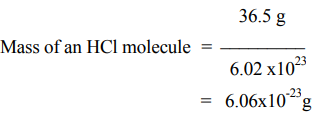1. The atomic weight of Cl is 35.5 amu, so the molar mass of Cl is 35.5 g/mol. Dividing 35.5 g (per mole) by 6.023 x 1023 gives the mass of one atom.

2. The molecular weight of HCl equal to the atomic weight of H, plus the atomic weight of Cl, (ie) (1.01 + 35.5) amu = 36.5 amu. Therefore 1 mol of HCl contains 36.5 g HCl