(a) The speed of electron in the nth orbit of a H-atom is given by
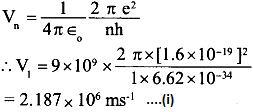
From the equation (i), it follows that the velocity of electron in an orbit of a hydrogen atom is inversely proportional to its quantum number (n).

(b) The orbital period of an electron in the nth orbit of a H-atom is given by

It follows that orbital period of an electron in H-atom is directly proportional to the cube of its quantum number.
