Whenever a single Lewis structure cannot describe a molecule accurately, a number of structures with similar energy, positions of nuclei, bonding and non-bonding pairs of electrons are taken as the canonical structures of the hybrid which describes the molecule accurately. These contributing structures are known as canonical forms. Resonance is represented by a double headed arrow. For resonance, the contributing structures should have same number of unpaired electrons, should have same atomic positions but differ in position of electrons, should have nearly same energy. Resonance in carbonate ion can be represented as :
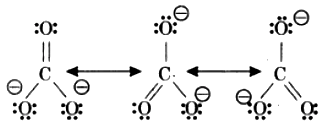
The actual structure is an average of these three resonating structures.