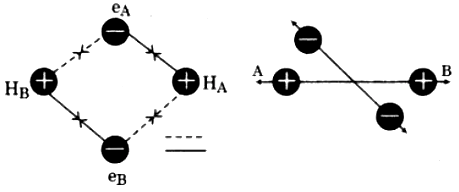
According to VBT, atoms combine so that their energy decreases and stability increases. Consider two hydrogen atoms A and B are approaching each other. When the two atoms are at large distance from each other, there is no interaction between them. As these two atoms approach each other, new attractive and repulsive forces begin to operate. These are: .
(i) Attractive force between nucleus of HA and electron of HB.
(ii) Attractive force between nucleus of HB and electron of HA.
(iii) Repulsive force between nuclei of both the atoms.
(iv) Repulsive force between electrons of both the atoms.
Experimentally, it has been found that the magnitude of new attractive force is more than new repulsive force. As a result, two atoms approach each other and potential energy decreases. Ultimately a stage is reached where the net force of attraction balances the force of repulsion and system acquires minimum energy. At this stage, two hydrogen atoms are said to be bonded together to form a stable molecule having the bond length of 74 pm. At this stage, the minimum distance between the two nuclei which is necessary for minimum energy and maximum stability is called bond length and the energy released in gaseous state by combination of two isolated atoms to form a molecule is called bond energy.