F2 can oxidize Cl– to Cl2, Br– to Br2, and I– to I2 as:

On the other hand, Cl2, Br2, and I2 cannot oxidize F– to F2. The oxidizing power of halogens increases in the order of I2 < Br2 < Cl2 < F2. Hence, fluorine is the best oxidant among halogens.
HI and HBr can reduce H2SO4 to SO2, but HCl and HF cannot. Therefore, HI and HBr are stronger reductants than HCl and HF.
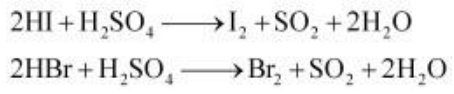
Again, I– can reduce Cu2+ to Cu+, but Br– cannot.

Hence, hydroiodic acid is the best reductant among hydrohalic compounds. Thus, the reducing power of hydrohalic acids increases in the order of HF < HCl < HBr < HI.