(i) Consider equation (1) and (2),
The concentration of A and B are constant. The rate remains the same for different concentrations of C. Hence, the order with respect to C is zero.
(ii) Consider equations (3) and (1),
The concentration of A and C are constant. The concentration of B is doubled, rate increases by 2 times. Hence order with respect to B is 1.
(iii) Consider equations (4) and (1),
The concentration of B and C remain constant. When the concentration of A is doubled, the rate increases by 4 times. Hence order with respect to A is 2.
Alternate method:
Suppose order with respect to A, B and C are α, β and γ respectively. Then the rate law at different concentration of A, B and C are
5.0 × 10-3 = k [0.010]α [0.005]β [0.010]γ …(1)
5.0 × 10-3 = k [0.010]α [0.005]β [0.015]γ …(2)
1.0 × 10-3 = k [0.010]α [0.010]β [0.010]γ …(3)
1.25 × 10-3 = k [0.005]α [0.005]β [0.010]γ …(4)
Dividing (1) by (2),
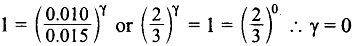
i.e., order with respect to C is zero.
Dividing (3) by (2),
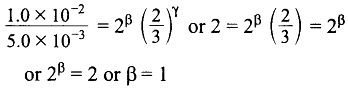
Dividing (1) by (4),
⇒ 4 = 2α or α = 2
Order with respect to A, B, C are 2, 1 and 0.